Treatment of splenic artery aneurysm
What is a splenic artery aneurysm?
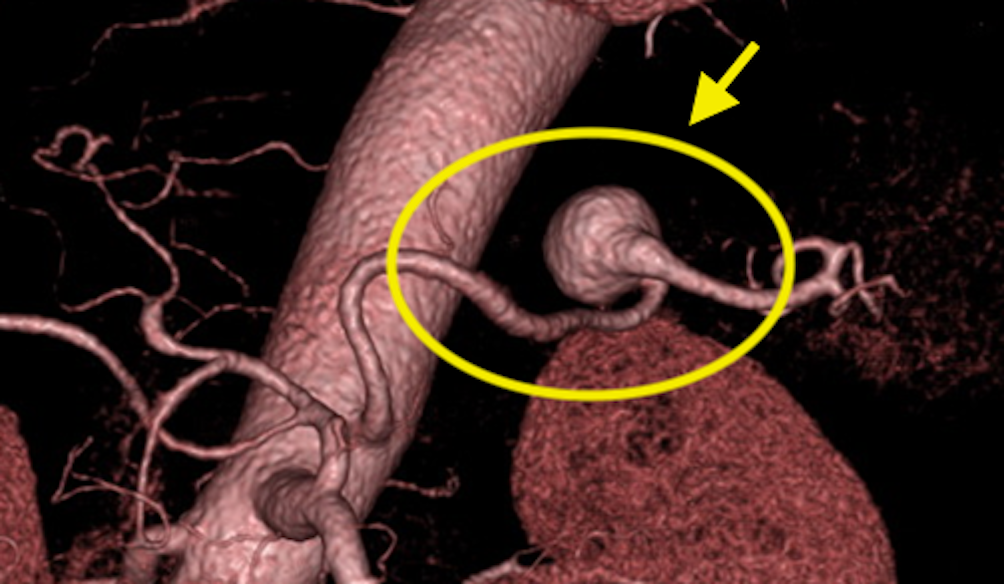
As the name implies, a splenic artery aneurysm occurs in an artery that branches from the aorta and supplies the spleen. (Fig. 1) Although it is a rare aneurysm, it is reported as the most frequent among visceral aneurysms and accounts for approximately 60% of visceral aneurysms. Although the rupture rate is assumed to be relatively small, it is life-threatening once ruptured, so an appropriate strategy should be demonstrated.
Treatment of splenic artery aneurysm
A standard threshold of operative intervention for a splenic artery aneurysm (SAA) was 2 cm in diameter. This value was not based on firm evidence, but on textbook descriptions or individual impressions, without having experienced or rarely heard of ruptured cases.
In a study of 70 cases of SAA reported by Sano M in our department, the factors that cause aneurysm expansion are (1) portal hypertension and (2) diameter ≥ 2 cm; severe calcification (called “egg shell”) is a factor for non-expansion. Therefore, we assume that the conventional surgical indication of ≥ 2 cm in diameter should be acceptable.
The first-line treatment should be endovascular treatment using a catheter, wire, and other devices. The target aneurysm and its entrance (afferent) and exit (efferent) arteries are embolized with coils to eliminate the blood flow toward the aneurysm wall. However, if the blood flow to a wide area the spleen is assumed to be interrupted due to embolization, open surgery is performed to reduce the aneurysm sac, bypass the aneurysm, or to perform splenectomy.
Relationship with pregnancy
It has been reported that SAA occurs four times more frequently in women than in men, possibly due to pregnancy. During pregnancy, hormones (estrogens and progesterone) secreted are thought to weaken the vessel walls, and increased circulating blood volume and abdominal pressure might promote the formation of aneurysms. The majority of ruptured cases were in the latter half of pregnancy, and if a patient with a SAA wishes to become pregnant, surgical interventions are required.
Portal hypertension
The blood from the spleen flows to the liver via the portal vein. When the liver is damaged due to various causes and becomes scarred, a condition called cirrhosis, a tendency for SAA to develop due to the stagnation of blood flow to the liver and an increase in pressure (portal hypertension) has been reported. There are reports that SAAs are frequently found in liver transplant recipients. Many patients underwent liver transplantation in our hospital, and the data revealed that portal hypertension was associated with SAA expansion.
A special factor called “Egg Shell”
Do you think that if the wall of the aneurysm is highly calcified, an aneurysm does not rupture easily? In general, the aneurysm wall is usually partially calcified, and we assume that calcification does not help prevent rupture, probably because the discrepancy between soft and hard areas can cause wall deterioration. However, SAAs often have a unique morphology with calcification which almost covers the entire aneurysm; this is called “eggshell” (Fig. 2).
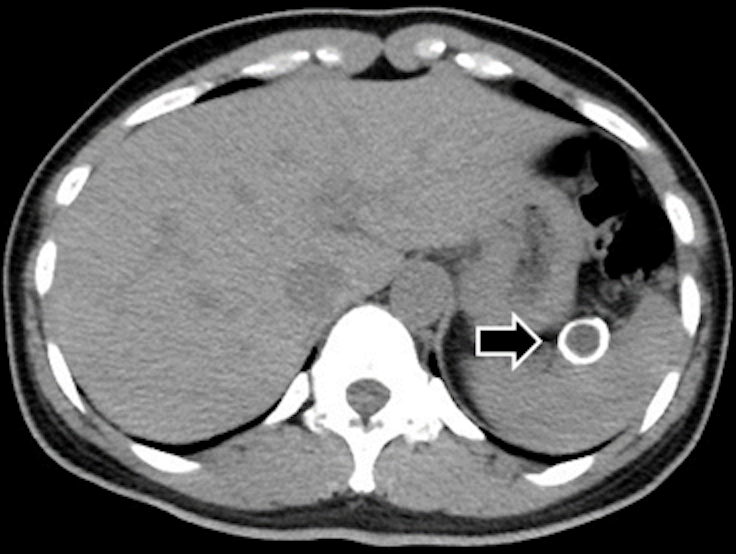
In our analysis, we found that eggshell is a factor that offsets risk factors such as diameter ˃ 2 cm and portal hypertension (Sano et al., Ann Vasc Surg 2019).
Tortuous and angulated splenic artery and the lesion of the aneurysm
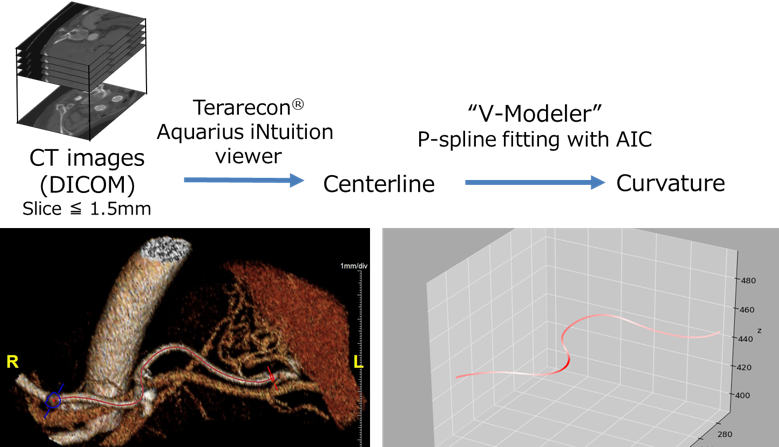
The splenic artery often has a tortuous and angulated morphology. Kimura et al. analyzed the three-dimensional (3D) shape of the splenic artery and quantified the flexion. In other words, they quantified the degree of winding and angulation as a numerical value (Fig. 3). The splenic arteries of the patients with SAA were more tortuous and angulated compared to those of normal people. They also found that the tendency was stronger in women (Kimura et al., Asian Cardiovasc Thorac Ann 2018).
Thus, the SAA has unique characteristics. Accordingly, the operative indication of 2 cm in diameter should be the standard, and we have to consider various patient factors including comorbidities and anatomy in the treatment planning.