What is “visceral aneurysm”?
What is visceral aneurysm?
There are rare aneurysms called “visceral aneurysms”. These aneurysms grow on the branches of the aorta, including the splenic artery, hepatic artery, renal artery, celiac artery, superior mesenteric artery, and pancreaticoduodenal artery (renal artery is sometimes excluded). Visceral aneurysms are often found incidentally on imaging modalities such as ultrasonography or computed tomography during a medical survey. Aneurysms have a risk of rupture. How dangerous is it?
For aortic aneurysms, the risk is size-dependent. However, the size of some visceral aneurysms is not necessarily associated with rupture risk. If you are diagnosed with “visceral aneurysm,” it might require immediate treatment. Renal artery aneurysm is assumed to have a smaller risk of rupture compared to that in other visceral aneurysms.
Guidelines for operative indication
In 2019, the guidelines for visceral aneurysms were published by the Society for Vascular Surgery (Chaer FA, et al. J Vasc Surg 2020; 72: 3 S-39 S). Standard values of aneurysm size for interventions on a true aneurysm (whose wall structure is maintained) are shown (Table 1). This guideline is just a reference. Some aneurysms are pseudo-aneurysms (those with unpreserved wall structures), some are rapidly growing, and some are ruptured or infectious, and are often indicated for surgery regardless of diameter.

Treatment of visceral aneurysms
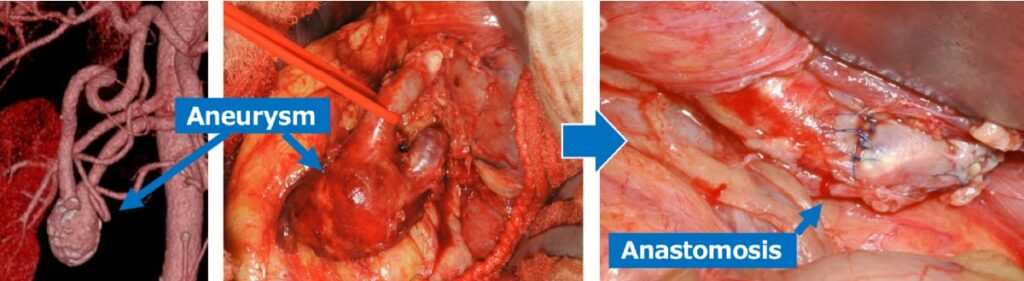
The first-line treatment is thought to be endovascular treatment, including arterial embolization. We embolize the target aneurysm and artery using a coil to eliminate blood flow. However, if embolization causes ischemic symptoms in the organs or a massive hematoma, open surgery with bypass is necessary (Fig. 1).
Simulation by 3D model
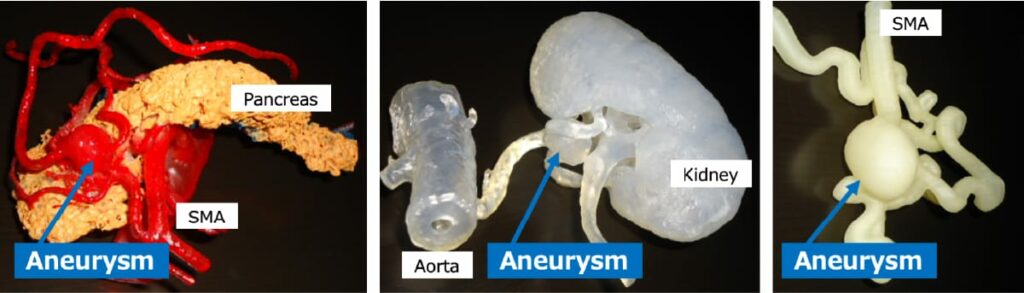
Since the lesion characteristics of these aneurysms vary widely, it is important for surgeons performing open surgery to be familiar with the surrounding organs. In my experience, even when I fully visualized the anatomy around the aneurysm with computed tomography (CT) images, the location was sometimes different from where I expected. In cases where the pancreas, liver, and other important organs adjoin the aneurysm, we create a 3D model of the aneurysm and adjacent organs using a 3D printer for the surgeons to understand and confirm the positional relationship (Fig. 2). A questionnaire for the surgeons revealed that they can perform the operation with an easy mind by imaging the anatomical situation in this manner.
Bizarre cause of pancreaticoduodenal artery aneurysm
Pancreaticoduodenal artery aneurysms, which form in the blood vessels around the pancreas, have unique characteristics. The pancreaticoduodenal artery forms an arcade with branches extending from both the celiac artery and the superior mesenteric artery, which are branches of the aorta (Fig. 3). It has been reported that patients with this aneurysm frequently experience stenosis and occlusion of the celiac artery. One of the causes of stenosis and occlusion is compression by the diaphragmatic arch ligament. Why does celiac artery stenosis cause pancreaticoduodenal artery aneurysm?
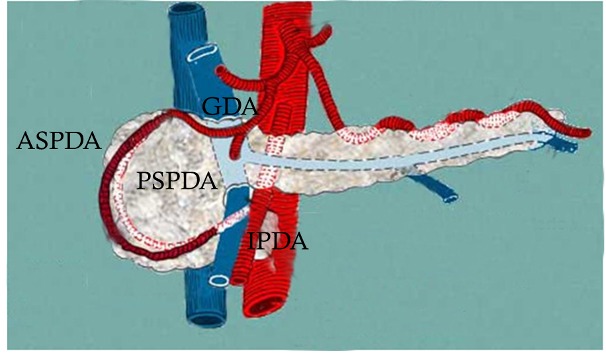
Hypothetically, the scenario is that (1) the blood flow from the celiac artery decreases, (2) therefore, the blood flow from the superior mesenteric artery increases, and (3) the hemodynamics of the arcade change significantly, resulting in arterial wall deterioration or remodeling and aneurysm formation. However, these aneurysms are rare; thus, it is difficult to prove this hypothesis clinically.
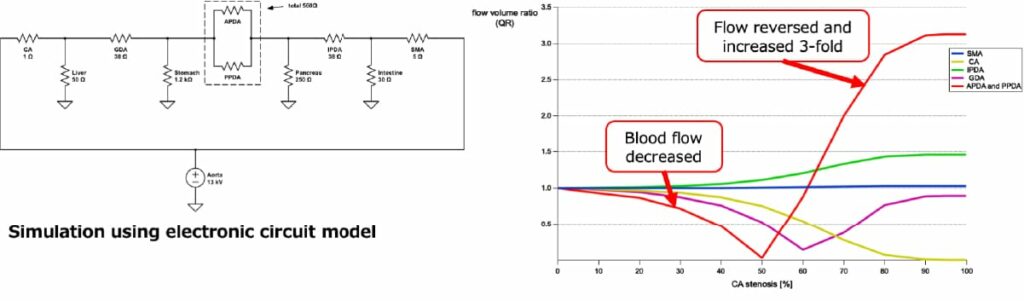
Therefore, we simulated using an electric circuit model. When the degree of celiac artery stenosis progressed from 0% to 99%, the blood flow decreased to 0% at 50% stenosis, and then increased 3-fold compared to the original flow rate (Fig. 4). It is not surprising that this dramatic change in blood flow might cause aneurysm formation. Considering the result of this simulation, once hemodynamics have been restored after treatment, there is no reason to perform additional interventions such as dilatation of the stenotic lesion. Therefore, we do not provide additional treatment for the celiac artery itself, such as dissection of the arched ligament after treatment of the aneurysm (or simultaneous surgery). Some surgeons believe that stenotic lesions should be fixed; however, our motto is to pursue the pathogenesis of the disease and treat it accordingly.
(Miyahara K, et al. Ann Vasc Dis. 2019 Jun 25;12(2))